Doxorubicin Hydrochloride Liposome Injection
type
Antineoplastic drugs
name
Doxorubicin Hydrochloride Liposome Injection
specs
10ml:10ml:20mg/80box
Drug approval number
国药准字H20233173
Indications
This product can be used for patients with low CD4 (<200CD4 lymphocytes/mm³) and AIDS-related Kapos's sarcoma (AIDS-KS) with extensive skin and mucosal visceral diseases.This product can be used as a first-line systemic chemotherapy drug, or as a second-line chemotherapy drug for the treatment of AIDS-KS patients with progression, and can also be used for patients who cannot tolerate the combination of two or more of the following drugs:vincristine, bleomycin and doxorubicin (or other anthracycline antibiotics).
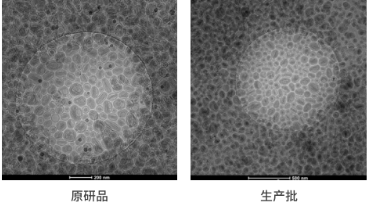
Irinotecan hydrochloride liposomal injection
type
Antineoplastic drugs
name
Irinotecan hydrochloride liposomal injection
Product progress
Marketing Approval Application
Indications
In combination with 5-fluorouracil (5-FU) and leucovorin (LV) in patients with metastatic pancreatic cancer who have progressed after treatment with gemcitabine.


 Corporate overview
Corporate overview Corporate Honors and Awards
Corporate Honors and Awards Core products
Core products